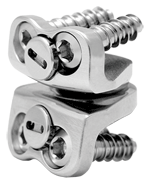
Houston, August 7, 2007 - Memorial Hermann Southwest Hospital medical staff physician Mohammad Etminan, M.D., has performed an artificial cervical disc replacement on a 37-year-old female suffering from degenerative disc disease in what is among the first such procedures to take place since the U.S. Food and Drug Administration (FDA) approved the PRESTIGE® Cervical Disc on July 16.
The first artificial disc commercially available in the U.S. for use in the neck, it gives some patients who are suffering from degenerative disc disease and intolerable neck and/or arm pain, the potential for motion, as well as another option for pain relief and function.
"This new surgical approach allows us to correct this painful problem so the patient can return to the activities of daily life.. It's a true medical milestone in spine surgery," said Etminan.
Kim Kulak is the first to be treated with the device in Houston. She was diagnosed with degenerative disc disease and believes the new device is her best choice, after suffering from chronic pain since September. "I don't want to take medications I would rather fix what's wrong with me and live normal."
The hour-long surgery should ease months of excruciating pain and allow Kulak to regain normal movement of the neck and spine.
Previously, a traditional motion-limiting spinal fusion procedure was recommended for the more than 200,000 cervical procedures performed each year to relieve compression on the spinal cord or nerve roots. This old procedure involved implanting an interbody graft and metal plate to rigidly fuse the vertebrae together, and required a three month recovery time.
That's not the case with the new procedure said Etminan. "Recovery time has been reduced from a few months to just ten days. The design of the disc better replicates the motion of a naturally functioning cervical spine."